

As a radioisotope tries to stabilize, it may transform into a new element in a process called transmutation. Elements with atomic numbers of 83 and less, have isotopes (stable nucleus) and most have at least one radioisotope (unstable nucleus). Radioactive isotopes are often called radioisotopes.Īll elements with atomic numbers greater than 83 are radioisotopes meaning that these elements have unstable nuclei and are radioactive. As the unstable nucleus changes, it gives off radiation and is said to be radioactive. So any elements above lead are unstable and can decay in some form or another, once a decay happens. In an unstable atom, the nucleus changes its configuration by giving off matter of energy to get to a balanced state. Lead has the highest atomic number for stable elements. The halflife of a radioactive element is the time needed for half of the material to decay The blue and orange points represent the original number of. However, each additional neutron may upset the balance and cause the atom to become unstable. Even if an atom has an additional neutron or two it may remain stable.
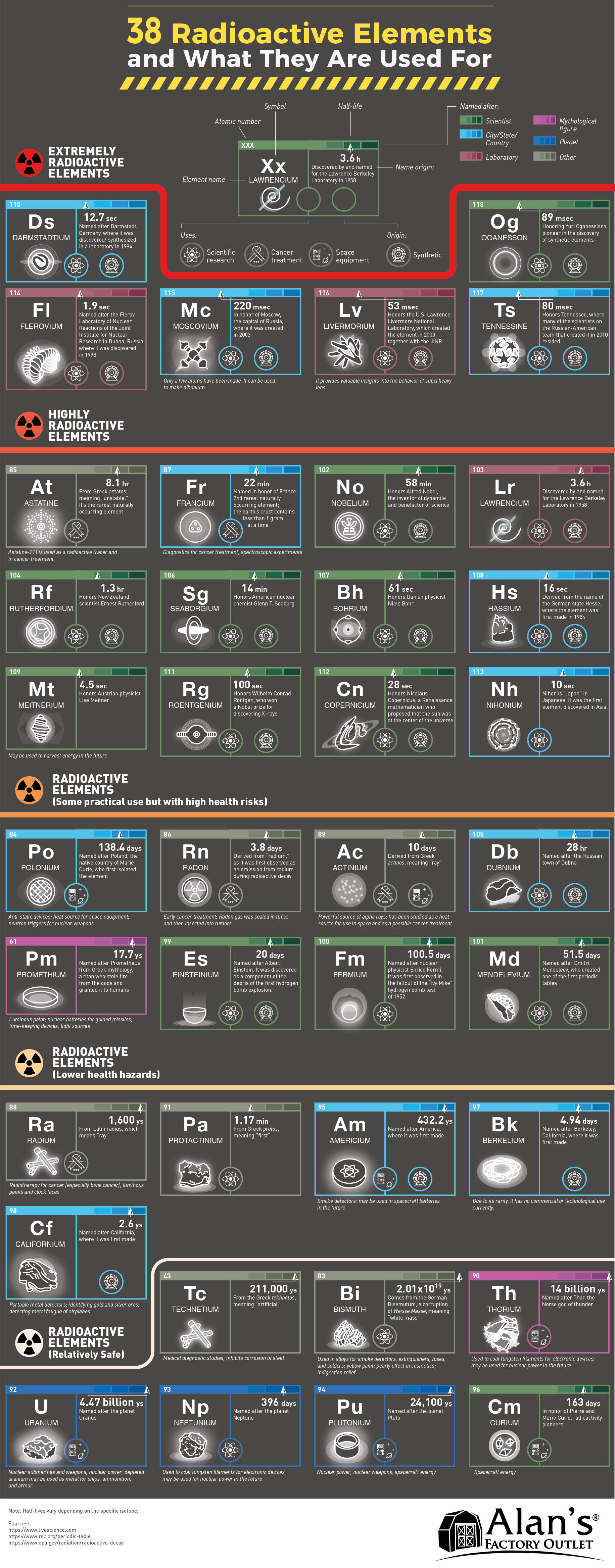
PERFECT FOR YOUR PERIODIC TABLE: Each of these light-up coasters feature a radioactive element. Stable atoms have a binding energy that is strong enough to hold the protons and neutrons together. Perfect for anyone who enjoys a bit of chemistry in their decor. In most cases, elements like to have roughly the same number of protons and neutrons because this makes them the most stable. The nucleus is held together by something called the binding energy. Because the like charges of the protons repel each other, these forces are always trying to push the atomâs nucleus apart. What is a radioisotope?Īs discussed in more detail elsewhere, isotopes are variants of an element that, while all having the same number of protons, have differing numbers of neutrons. Radioactivity is the release of energy or ejection of matter that results from changes in the nucleus of an atom. Buried at the bottom of the periodic table. The unstable nucleus will emit energy in some form and is said to be radioactive. A UTEP chemist has been awarded 195,000 from the Welch Foundation to research understudied radioactive elements. THE Czechoslovak newspapers reported on July 5 that an element of higher atomic weight than uranium has been discovered in Joachimsthal pitchblende by Dr. Explain how a radioisotope differs from an isotope.Ītoms with unstable nuclei are constantly changing as a result of the imbalance of energy within the nucleus.



 0 kommentar(er)
0 kommentar(er)
